-
FDA Declines Recro's IV Meloxicam Application
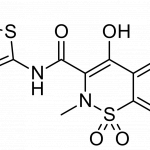
Thursday, May 24, 2018 - 10:11am | 345Read More...Recro Pharma Inc (NASDAQ: REPH) needs an analgesic of its own. The firm announced Thursday morning that the U.S. Food and Drug Administration declined to approve its IV meloxicam application for non-opioid pain relief "in its current form." The Complete Response Letter acknowledged...
-
Exclusive: Recro Pharma CEO Talks Meloxicam Non-Opioid Product Ahead Of APS Annual Meeting
Wednesday, May 17, 2017 - 11:15am | 572Read More...Recro Pharma Inc (NASDAQ: REPH) recently announced positive Phase 3 safety data for its palliative drug, IV Meloxicam, and CEO Gerri Henwood is eager to take the non-opioid, preferential COX-2 inhibitor to the next level. The company looks to file a New Drug Application with the Food and Drug...
-
Recro Pharma CEO Discusses Strong Results In Non-Opioid Pain Treatment Study
Thursday, December 1, 2016 - 11:49am | 619Read More...Recro Pharma Inc (NASDAQ: REPH) is a micro-cap specialty pharmaceutical company focused on the development of non-opioid products for the treatment of serious acute pain. After a very volatile year, the stock is down 9.4 percent, although it has gained almost 14.5 percent in the last month, largely...