-
Rocksteady Lifts NDA On 'Suicide Squad: Kill The Justice League' Alpha: 'Please Feel Free To Talk And Write'
Friday, January 12, 2024 - 4:54pm | 441Read More...Video game developer Rocksteady has lifted a portion of the non-disclosure agreement (NDA) on the closed alpha for "Suicide Squad: Kill the Justice League" following media previews. While publications expressed reservations about the game, Rocksteady — owned by Warner Bros....
-
Alkermes Falls 20% After FDA Refusal To File Letter
Monday, April 2, 2018 - 8:38am | 396Read More...Shares of Alkermes Plc (NASDAQ: ALKS) lost more than 20 percent early Monday morning after the U.S. Food and Drug Administration said it won't review the company's therapy ALKS-5461. What Happened Alkermes, which mostly focuses on treatments for central nervous system diseases, said...
-
PTC Therapeutics Moves On Old Report
Thursday, June 1, 2017 - 12:13pm | 207Read More...PTC Therapeutics, Inc. (NASDAQ: PTCT) plunged as low as 4 percent off Thursday’s highs as traders circulated a report citing termination of the firm’s 2A ataluren study for Duchenne and Becker muscular dystrophies. However, a company spokesperson clarified that this was “very old...
-
TherapeuticsMD Results 'Worse Than Expected'
Monday, May 8, 2017 - 8:45am | 453Read More...Shares of TherapeuticsMD Inc (NYSE: TXMD), a healthcare company that focuses on creating and commercializing products for women, fell more than 11 percent Monday morning after the company announced it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration. The...
-
AcelRx CFO Shares Update On DoD Relationship And More After FDA Accepted NDA For DSUVIA
Tuesday, February 28, 2017 - 6:45pm | 774Read More...Micro-cap specialty pharmaceutical company AcelRx Pharmaceuticals Inc (NASDAQ: ACRX) announced Monday the FDA accepted its New Drug Application for DSUVIA, its treatment for moderate-to-severe acute pain in medically supervised settings. In addition, the federal agency set a PDUFA date of Oct. 12,...
-
Getting Into The Drug Business: From NDAs To PDUFA Dates
Tuesday, September 27, 2016 - 2:20pm | 665Read More...The strongest catalysts for a pharma stock are the various milestones on the road to FDA approval for new drugs. For new investors, they’ll often see a stock rise astronomically, but, when they go to make sense of the reasons for the jump, they’re greeted with unfamiliar acronyms. Let...
-
Clovis Oncology Falls 8%: Here's What You Need To Know
Friday, April 8, 2016 - 2:11pm | 320Read More...Shares of Clovis Oncology Inc (NASDAQ: CLVS) lost more than 8 percent on Friday after the company confirmed that the FDA posted briefing documents ahead of the Oncologic Drugs Advisory Committee (ODAC) meeting to discuss accelerated approval of the New Drug Application (NDA) for rociletinib. The...
-
Puma Biotechnology Analysts Are Getting Nervous, But Still Going Long
Tuesday, March 29, 2016 - 11:51am | 543Read More...Puma Biotechnology Inc (NYSE: PBYI) shares are down more than 26 percent on Tuesday and 29 percent since Monday's open. The stock's plunge comes on the back of news that the biotechnology company has plans to delay its NDA (New Drug Application) filing after meeting with the FDA (U.S. Food and Drug...
-
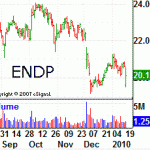
Not Enough Room in This Town for Both ENDP and WPI
Tuesday, January 19, 2010 - 11:44am | 120Read More...Slow down a minute Endo Pharmaceuticals Holdings Inc (NASDAQ: ENDP) - there’s already a generic Lipoderm skin patch like the one you just submitted an NDA (new drug application for). At least that’s the story from Watson Laboratories Inc. (NYSE: WPI), which recently notified Endo Pharmaceuticals...